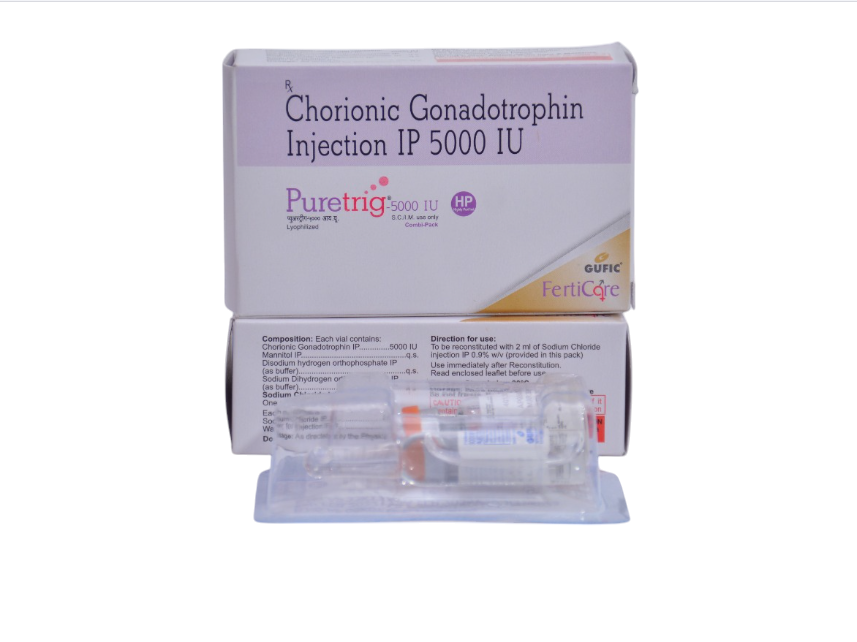
Chorionic Gonadotrophin 5000 IU 20 Vials
€180
Hormonal drug
In stock
Chorionic Gonadotrophin 5000 IU 20 Vials
Product Details
Chorionic gonadotropin 5000 IU 20 vials
Chorionic gonadotropin 5000 IU in 1 vial is a hormone that is used to trigger ovulation in women suffering from infertility and in men with low sperm survival, and is also used to stimulate Leydig cells to accelerate the formation of testosterone. . This product is available in a vial containing 5,000 IU of hormone, which is injected.To use this drug, first you need to mix the contents of the vial with the sterile liquid included in the package of the drug. Then, using a syringe, make an injection according to the instructions of your attending physician. It is important to carefully follow the instructions provided by your doctor to ensure the safe and effective use of this hormone.
Indications for use
For women:
- induction of ovulation in infertility due to anovulation or impaired maturation of follicles;
- preparation of follicles for puncture in programs of controlled ovarian stimulation (for assisted reproduction techniques);
- support of the luteal phase (including during the period of controlled ovarian stimulation with assisted reproductive technology (ART)) with the use of gonadotropin-releasing hormone analogues or other means to stimulate ovulation in female infertility as a result of anovulation due to insufficiency of endogenous estrogens (ovarian insufficiency of the WHO classification group I).
For men:
- hypogonadotropic hypogonadism;
- infertility associated with idiopathic dyspermias;
- delayed puberty due to insufficient gonadotropic function of the pituitary gland;
- cryptorchidism, not caused by anatomical obstruction.
Contraindications
• hypersensitivity to human gonadotropins or to any component of the drug;
• identified or suspected tumors dependent on sex hormones (ovarian cancer, breast cancer and uterine cancer in women, and prostate cancer, breast cancer in men);
• organic lesions of the central nervous system (CNS) (tumors of the pituitary gland, hypothalamus);
• deep vein thrombophlebitis;
• hypothyroidism;
• adrenal insufficiency;
• hyperprolactinemia.
For men (optional):
infertility unrelated to hypogonadotropic hypogonadism;
For women (optional):
• improper formation of the genitals, incompatible with pregnancy;
• fibrotic tumor of the uterus, incompatible with pregnancy;
• history of ovarian hyperstimulation syndrome (OHSS);
• Polycystic ovary syndrome (PCOS);
• primary ovarian insufficiency;
• bleeding or spotting from the vagina of unclear etiology;
• pregnancy and breastfeeding.
Storage of the drug in an undissolved form at a temperature of 12-25 C in a dissolved 2-8 C
Manufacturer Gufic Biocience (India)
You May Also Like